| Atomic Structure | ||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| What is an Atom? | Does anybody know? Has anybody seen an atom? How big is it? How do we know? Is there Similitude between Atoms and Galaxies? Are there more Atoms in the Periodic Table that we do not know of? |
Where did you see your first atom? Is the atom a particle? Is it a wave? Is a Photon an atom? |
||||||||||||||||||
| Electronic Structure, VSEPR | n: principal quantum number: 1,2,3,4 l: 0s, 1p, 2d, 3f orbital. S: 0, P: -1,0,+1 m: for p subshell (-1, 0, 1)... s: spin: angular momentum s p d f 1 2 2 3 3 3 4 4 4 4 5 5 5 5 6 6 6 7 7 |
|
||||||||||||||||||
| Effective nuclear charge | #protons - nonvalence electrons. Higher nuclear charge = smaller atomic radius. |
|||||||||||||||||||
Periodic Trends The Periodicity of Elemental Structure: Electron Affinity vs Electronegativity vs Ionization Energy |
The Periodic table is not a square table ; Atomic Radius increases down a column and to the left in a period. Ionization Energy increases to the right in a period and up in a column. Helium has highest ionization energy. Electron Affinity: Increases to the right in a period and up in a column. Generally it increases up in a column, ex Cl vs F Electronegativity: Increases up a column and to the right. FOClNlBrISCH:decreasing order of electronegativity Electron Affinity follows trend of Electronegativity, generally increasing as a more stable structure is formed when an electron is gained. Ionization energy is the energy required to remove an electron from an atom. Electronegativity with respect to an atom in a molecule. Lattice energy is the energy released when a crystal is formed from its constituting ions. e.g. Na+ Cl- -> NaCl(s). The ionic bond is stronger as the charges between atoms is larger. Al(OH)3 is insoluble in water because of large lattice energy Acidity increases to the right in a period and down a column. Is related to the stability of the anion. As the size of the anion is increased charge can become delocalized easier. |
|||||||||||||||||||
| Periodic Exceptions | Cl has higher electron affinity but lower Electronegativity than F because of electron pair repulsion in the valence shell. Transition metal: When d-block elements form ions, the 4s electrons are lost first. Vanadium has a higher melting point than Cr. Generally melting point is increased to the right within a period because of london/induced dipole forces. V:[Ar] 4s2 3d3; V3+[Ar] 3d2 Cr has a half filled d level [Ar] 4s1 3d5 Cu: [Ar] 4s1 3d10 Au: has only one valence electron: Ag: [Kr] 5s1 4d10 |
21Scandium and 22Titanium: 1s2 2s2 2p6 3s2 3p6 4s2 3d1 , 1s2 2s2 2p6 3s2 3p6 4s2 3d2 |
||||||||||||||||||
| Bonding | Charge, Ionic and Polyatomic Ions, Ionic Strength; Hydrogen Bond, Electronegativity, NH4+, PO4-3, SO4-2, ate-ite |
|||||||||||||||||||
| Covalent Bonding | Hybridization, Geometries sp3, sp2, sp | Carbocation has sp2, trigonal planar geometry | ||||||||||||||||||
| Hydrogen Bonding: Dipole Moments | FON: A hydrogen bonded to a electronegative halogen can Hydrogen bond with another electronegative halogen. This can occur intramolecularly within short distances, around 1 C-C length. |
|||||||||||||||||||
Acid and Bases: Reduction Oxidation Reactions: Lewis Acid, Bronsted Acid, Lewis Base, Bronsted Base, Buffer, K, pK, Strong vs Weak Acids, Titration, Hydrolysis, Electrolytic Cell, Anode Cathode, Galvanic vs Electrolytic Cell, Reduction Potential. Disproportionation |
Arrhenius Acids yield H+. If a substance accepts a pair of electrons to form a covalent bond it is condsidered a Lewis Acid. Reducing Agents: Reductor: Reducer: looses electrons: LiAlH4, H2. Metals act as electron donors giving off ions in solution.Then it is said that the metal has been oxidized. Fe(s)-> Fe++ + 2e-. Substances that are oxidized act as REDUCING AGENTS. Reducing Agents are oxidized. Oxidizing Agents are Reduced. Oxidant = Oxidizing Agent = Oxidizer Reductant = Reducing Agent = Reducer. Reducers gain Electrons given by the Reducing Agent that has been Oxidized (donating electrons). LiAlH4 + 4 H2O → LiOH + Al(OH)3 + 4 H2 CARDIO Base: Charge-Atomis size-Resonance-Dipole Induction-Orbital: more negatively charged species are more basic, larger atoms with negative charges are more stable weaker bases, more resonance more stability, sp3 more basic than sp2... |
NADP+ + 2H2O -> 2NADPH + 2H+ +CO2 NADP+ accepts electrons from water. This is a reduction. NADPH is the reducing agent. NADH IS NOT NADPH. Acidity and Melting Point are not related. Larger atoms are more acid because can form more stable anions. |
||||||||||||||||||
Redox
|
Oxidation: Gain of O, Loss of e-, Loss of H+
|
|||||||||||||||||||
Arrhenius Definition
|
Arrenius Acids : Donate H+ Arrhenius Base: Accepts H+ Compounds ionize in solution (water only). |
|||||||||||||||||||
Lewis Definition
|
Lewis Acid accepts e- pair: Electrophile: Trigonal Planar BR3 with empty p orbital. H+ is a LA. H+ + NH3 → NH4+ . Carbonium is electrophilic thereby a strong LA. Strong Lewis acids are electron deficient. Strong Lewis Bases are electron rich. Lewis Acid Base Pair Lewis Base donates NONBONDING e- pair: H-, F-; are NUCLEOPHILES |
Basic Amino Acids: Lys, Arg, His. Conjugates of Strong Acid/Base have no effect on pH:
|
||||||||||||||||||
| Bronsted Lowry | Acids increase H3O+, Bases Increase -OH | |||||||||||||||||||
| Conjugate Acid/Base Pairs | To find the conjugate base of an acid, take off a H+ HCl is a strong acid. Cl-, its conjugate base has no effect on pH. Acids dissociate completely. The more stable is the anion the more acid is the molecule. NH4Cl: NH4+ is the conjugate acid of weak base NH3 Weak base NH3, conjugate acid NH4+ strong acid. acid + base = conjugate acid + conjugate base |
Strong Acids: HNO3, H2SO4, HCl, HClO4, HBr, HI SuperStrongAcids: HFSO3-SbF5 other scales beside pH are used to measure SSA. Corrosiveness NOT EQUAL to Strength = HF |
||||||||||||||||||
| Ka vs pKa | COOH has a pKa of 4 - 5 The lower the pKa the higher the Ka. Low pKa are acidic. High pKa are basic. When pKa and Ka are equal the compound is in equilibrium and the slope of titration curve is linear. |
|||||||||||||||||||
ELECTROCHEMISTRY |
ELECTROCHEMISTRY From Nerst to Current Trends
|
|||||||||||||||||||
Electrostatic Energy Lattice Energy kQq/r vs F =k(q1q2)/r^2 ΔU = ΔH - p ΔV Gauss Law: I = EAcos(-) F = qvB sin(-) F = qE |
Coulomb's law k = 9E9 Nm^2/C^2 Electric field lines move out of the positive charge and into negative charges. V=J/C A dipole aligns so that the positive lines are "into the negative and out of the positive". The positive end of the dipole will be aligned with the electric field lines. Charge moving in a circle: F = qvB = mv^2/r ; if qvB> mv^2/r the charge is spiralling in. if qvB<mv^2/r the charge is curving. Current Carrying wires: F = qvB sinθ = (it)vB sinθ = (it)(L/t)B sinθ = iLB sinθ A negative Force means that charges are attracting. |
http://farside.ph.utexas.edu/teaching/em/lectures/node56.html | ||||||||||||||||||
Current |
CURRENT TRENDS: Conventional Current Flow is Opposite to Electron Current Flow. Arbitrarily the current flows out of the short battery symbol into the circuit. The Current in a Parallel Circuit is Split at Ramifications like water flow. The current in a Series Circuit is Steady. What drops is the voltage (like water pressure), as a loss to Impedance.
|
Resistors in series have the SAME current. | ||||||||||||||||||
|
Electrolytic Cell |
E(cell) > 0, the reaction is spontaneous and happens in a galvanic cell. Faraday's law E(cell) = E(oxidation) + E(reduction) |
|||||||||||||||||||
| Galvanic = Voltaic Cell | Two pure metals connected by a salt bridge. Oxidized species + e- = Reduced Species Metal cations induce reduction of metals in other cell. So the more positive Oxidation Potential Metal gives off electrons from the Anode to the other metal Cathode . Zn + Cu2+ → Zn2+ + Cu . In a galvanic cell of Zn and Cu electrodes. The Zinc electrodes looses mass as electrons go to Cu2+ and Zn++ goes into solution. |
Ionization Energy increases to the left and down a column. + | ||||||||||||||||||
| Difference b/w Electrolytic and Galvanic Cell | ||||||||||||||||||||
| Capacitor | U = (1/2)CV^2 | |||||||||||||||||||
| Laws | Ideal Gas, Boyle, Charles, Avogadros: Henry, Raoult, Arrhenius, Hess, Zeroth, First Law, Second Law, Rate Law, Le Chatelier, Faraday, Newton's First, Newton's Second Law, Dalton's Law | |||||||||||||||||||
Kinetic Theory of Gases k = R/Na Partial Pressure, Mole Fraction STP: 0C, 1atm |
Boyles law: PV = k: inverse proportionality P1V1 = P2V2 Charles law: V/T = k: direct proportionality: V1/T1 = V2/T2 Avogradro's Law: V1/N1 = V2/N2 Dalton's Law: p = xP also vi = V * pi/P Henry's law pi = k ci: pi is the partial pressure of the solute. "At a constant temperature, the amount of a given gas that dissolves in a given type and volume of liquid is directly proportional to the partial pressure of that gas in equilibrium with that liquid." Raoult's law: p = p* x; p* is the vapor pressure of pure component. Raoult vs Henry: Henry is more related to solute, Raoult to solvent: When the solvent is in a great quantity the constant of proportionaliy is the partial pressure of the solvent. |
|||||||||||||||||||
| Real Gas Law, Van der Waaaals | 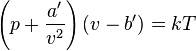 |
|||||||||||||||||||
| PRESSURE AND MASS EFFECTS | Molarity: kg / L Molality: mol / L Normality: meq / L Molar Volume at 0C and 1atm = 22.4L/mol |
f = Av
|
||||||||||||||||||
| Mass Effects Return Home | Surface Tension | |||||||||||||||||||
Colligative Equations
|
Solubility vs T, P. Osmotic Pressure: Solvent moves across a semipermeable barrier from high concentration to low concentration. The pressure difference across the chambers is the osmotic pressure. Chaotropic agents: increases solubility of non polar. (Alcohol, Urea, Lithium acetate). Denature proteins by increasing hydrophobic solubility. Kosmotripic increase water-water interactions. Stabilizes intermolecular protein interactions thereby preventing aggregation. (Mg2+, Li+1, Zn2+), mostly small positive ions). Ammonium sulfate. |
|||||||||||||||||||
Surface Tension |
Mechanical Properties of Liquids |
|||||||||||||||||||
| Solubility | Ksp All 1A metal salts are water soluble All NH4+ salts are water soluble All NO3--, ChH3O2-, ClO4- , are water soluble. KNO3 SO4 -2 sulfate PO4 --- phophate Complex Ion formation, solubility Common Ion effect Silver salts are insoluble in water with the exception of silver nitrate, silver acetate and silver sulfate. Chlorides Bromides Iodides, are soluble with the exception of AgCl2, PBCl2, HgCl2 |
Base Solubility: Ca(OH)2 is soluble. Group 1 and NH4+ salts are soluble Group 2 MgO, metal oxides Insoluble. Sparingly soluble salts derived from weak acids tend to be more soluble in an acidic solution. Which of the following insoluble salts—AgCl, Ag2CO3, Ag3PO4, and/or AgBr—will be substantially more soluble in 1.0 M HNO3 than in pure water? Answer: Ag2CO3 and Ag3PO4 Good Reference: http://chemwiki.ucdavis.edu/Wikitexts/UC_Davis/UCD_Chem_2B/UCD_Chem_2B%3A_Larsen/Unit_IV%3A_Chemical_Equilibria/Chapter_17/17.4_Solubility_and_pH |
||||||||||||||||||
| Buffers | Tritration Curve effect | |||||||||||||||||||
| Ksp | Ksp describes saturated solutions. BaC O 3(s) ---- Ba 2+(aq )+C O 2− 3(aq ) Ksp= [Ba2+][CO32-] = 5.1 x 10-9 |
|||||||||||||||||||
Heat Effects Conduction, Convection Radiation, Work, Rankine Scale, Absolute Scale, Conservation, Decay, Half Life |
Enthalpy Hess Bond Dissociation Heat Endo, Exo, Enthalpy, Heat Capacity, Specific Heat, Entropy, Gibbs Free Energy, Spontaneity, Heat of Fusion, Heat of Vaporization Zeroth Law, First Law, Second Law. |
|||||||||||||||||||
| Heat of Hydration | Thermodinamically favorable. Solvation releases heat. First heat is required (ENDOTHERMIC) to break up an ionic crystal lattice. The hydration step is exothermic. |
Think of Dissolving Salts, is it Exo/Endo Thermic? | ||||||||||||||||||
| Heat of Combustion | Energy released when a compound is completely combusted with O2 under 1atm, 298K. Ring Strain and Transanular effects result in increasd Heat of Combustion. Ciclohexane has lowest heat of Combustion per CH2 group. o |
Reference: | ||||||||||||||||||
| Heat of Formation | Standard States 1atm of gas, or elemental solid. at constant T 298K, P 1atm. Sum of all the bonds broken and all bonds formed from elemental atoms or molecules in their std state. |
|||||||||||||||||||
| Le Chatelier | In an equilibrium reaction mass of a species, temperature, or pressure can shift the equilibrium concentrations to favor an increae of products or reactants. | Reference: http://www.citycollegiate.com/chemical_equilibrium8.htm | ||||||||||||||||||
| Spontaneity | ΔG is negative. Standard Reduction Potential is positive E0. DeltaG = -RTln(K); DeltaG = -nFE |
|||||||||||||||||||
| Kinetic Effects | ||||||||||||||||||||
| Catalysis, Enzymes, | Reaction Order, Reaction Rate Rate Determining Step
|
enzyme-catalysed reactions display saturation kinetics. increasing substrate concentration means an increasing rate at which the enzyme and substrate molecules encounter one another. Enzymes lower Activation Energy. Enzymes do not change the Thermodynamics of a Reaction. |
||||||||||||||||||
| QUANTUM MECHANICS | Alpha particles: He nucleus: 2 protons, 2 neutrons Beta particles: +/- Beta Decay: neutron decomposes. Beta decay is usually B(-) B(+): Nucleus converts a proton into a neutron and a positron.The positron is liberated. Atomic number decreases. Occurs when the isotope has too few neutrons. B(-): electron is liberated. Atomic number is increased. Neutron is decomposed into electron and proton. Electron is referred to as B particle. Electron Capture EC: electron meets proton and is converted into a neutron. Mass number stays the same but proton number is reduced. Element change. Gamma Radiation: Eenergy emmitted as electromagnetic radiation. |
Alpha particles are very destructive. 4/2. They are quickly absorbed by skin. c = 3(10)^8 m/s |
||||||||||||||||||
Wave Duality, Wave Properties, Amplitude, Intensity, Superposition, Resonance, Diffraction, Refraction, Harmonic Motion, Pendulum, Differential Movement Materials Sciences Solids, Density, Thermal Expansion, Viscosity, Magnetism, Force Direction, Coulomb, Ampere, Field Line, Charge Distribution, Insulation,
|
L= Iw moment of inertia times angular velocity around axis of rotation tangential velocity = rl: product of radius and angular velocity
Centripetal force F = -mv^2/r Momentum p = mv; m = Δ P / Δ t Impulse I = Ft Hook's law F = -kx |
Momentum is always conserved: in elastic and inelastic reactions momentum is conserved. In Inelastic collisions KE is NOT conserved. KE is lost.
|
||||||||||||||||||
| SOUND | ||||||||||||||||||||
| Sound Waves | Transverse Wave: Displacement of medium is perpendicular to direction. A transverse wave can only occur in the surface of a liquid, not in its body. TW can occur in solids. Longitudinal Wave: Displacement of medium is along direction of motion. Sound can be represented as a Longitudinal wave. Light can be represented as a Transverse Wave. |
Spreading Equation Δθ = λ / d | ||||||||||||||||||
| Half Life | N= No(1/2)^(t/t(1/2)) | |||||||||||||||||||
Current, Voltage, Resistance, Ohms, Resistivity, Capacitance Circuits, Capacitor, Dielectric, Power, Circuits in Series, Circuits in Parallel |
V = IR I = Δ Q / Δ t P = VI = RI^2 RMS I RMS V B = [Ns/Cm] = Force/ ChargexVelocity |
Ohms Law is used to calculate Voltage Drop across a resistor. | ||||||||||||||||||
| How do you know the Magnetic Field is Perpendicular to the Electric Field? | ||||||||||||||||||||
| Applied Effects —°µ | ||||||||||||||||||||
| Hydrostatic Pressure, Surface Tension, Bernoulli's Equation, Flow Profiles | ||||||||||||||||||||
| Normal Force, Vectors | Collisions: Perfectly Inelastic p = (m1 + m2) v |
|||||||||||||||||||
| Radians | Theta = arc length/ radius 1arcsecond=3600radians 2pi/360 = |
Refer to spreading equation of light and sound. The spreading is proportional to the wavelength of the emitted wave over the distance of the orifice of emission. | ||||||||||||||||||
| Power | Units: Watts: [W] P = VI = V^2 R | |||||||||||||||||||
| Energy Work | Units: Joules: [J] | |||||||||||||||||||
| Potential Energy | PE=mgh, PEspring=kX^2/2, PEg= -GmM/r ΔPE=qDeltaPHI: electric lines point in the direction of the lower electric field..
|
|||||||||||||||||||
| Force | Fspring = -kx | |||||||||||||||||||
| Waves | Light, Polatization, Refraction, Diffraction, Doppler Effect | |||||||||||||||||||
| Light | Interference, Young's double slit, laser, Color and Energy Angle of Incidence = Angle of Reflectio n with respect to the NORMAL. The angle is often referred with respect to the surface. Index of Refraction n = c / v. n1 sin( 1) = n2 sin(2). Internal reflection occurs when light travels from a medium of lesser light speed to greater light speed (greater n to less n) Because the index of refraction of red and blue light is different the eye cannot focus on red and blue light in the same place.
|
Visualize Water. | ||||||||||||||||||
| Magnetism | Direction and force of charge in magnetic field. | |||||||||||||||||||
| Resistance | Resistance is added in series. | |||||||||||||||||||
Voltage
|
Parallel Voltages are equal. Voltage is a Potential Difference V = I R |
Galvanic and Voltaic cells are the same. Electrolytic cells drive nonspontaneous reactions. |
||||||||||||||||||
Circuits
|
Current, Voltage, Resistance, Resistivity p = RA/L, Capacitance RMS Voltage, RMS current. |
|||||||||||||||||||
Capacitor
|
Capacitors in series are 'parallel added'
|
|||||||||||||||||||
| Optics | (1/f) = (1/i) + (1/o); Magnification: M= -i/o; Focal length (f) is 1/2 radius of curvature: f = r/2; Lens Power: [Diopters] = 1/f Snells law: n sin () = n sin () Concave Mirror: Real (always Inverted) image small, same, larger to underfined, Virtual larger image Convex Mirror: Virtual Erect smaller image. The Cocave Lenses are Diverging.focal length is (-) for a diverging lens. Diverging Lenses form only Virtual Images. Virtual Images are upright so their magnification value is (+)
Diverging. Convex mirrors and Concave lenses NEVER form Real Images. Real images can be cast on a Screen? |
f is positive for a concave mirror and negative for a convex mirror. All real images are inverted and all virtual images are upright. How does the image of a Concave Mirror change as you move closer? http://mcat-review.org/light-geometrical-optics.php |
||||||||||||||||||
Optics: Eye Correction |
Myope: Nearsighted: cannot see far away. Light focuses in front of retina. Divergent lenses are needed. Hypermetropy: Farsighted: cannot see close. Light focuses behind retina. Convergent lens needed. We loose Acccomodation as we age. Accomodation is the ability to |
|||||||||||||||||||
| Converging Lens | http://www.acs.psu.edu/drussell/Demos/RayTrace/Lenses.html | |||||||||||||||||||
| ORGANIC CHEMISTRY | ORGANIC CHEMISTRY | ORGANIC CHEMISTRY | ||||||||||||||||||
| Nomenclature of Alkanes | 1. Take longer chain 2. Number the carbons of the parent chain from the end that gives the substituents the lowest numbers. When compairing a series of numbers, the series that is the "lowest" is the one which contains the lowest number at the occasion of the first difference. If two or more side chains are in equivalent positions, assign the lowest number to the one which will come first in the name. 3. If chains of equal length are competing for selection as the parent chain, then the choice goes in series to:
|
GOOD REFERENCES http://www.acdlabs.com/iupac/nomenclature/79/r79_36.htm http://www.iupac.org/ http://butane.chem.uiuc.edu/cyerkes/Chem104A_BFA05/Genchemref/nomenclature_rules.html |
||||||||||||||||||
| Hybridization | sp is 50% s character which is less energetic than p. This results in increased acidity. SP2 Hibridization: Trigonal planar geometry. Free p electron. Nitrogen in a cycle. Nitrogen in a cycle has the free pair projected outside the plane in trigonal planar geometry, sp2 hibridization. SP Hibridization examples: triple bonds. |
Exceptions: BH3 is sp2 hybridized. CO2: sp with 180' |
||||||||||||||||||
| Formal Charge |
FC = V- (N + B/2); e.g. NH4+: Nitrogen has a +1 formal charge (5- (0 + 8/2) = +1) Oxygen in NO2- : 6 - (4 + 4/2) = 0 |
Formal Charge of Phosphoric Acid.P=0, hibridized sp3 FC = Valence - (alone bond pairs - half of bonding electrons) 5-(0+10/2) = 0 |
||||||||||||||||||
| Degrees of Saturation | (C2n+2 - H) / 2; example BuckyBall C60: (122 - 0) / 2 = 61 Nitrogens count as 0.5C Oxygen does not count. Halogens count as Hydrogens. Nit |
Additional Equations exist that give the same result for degrees of unsaturation. Shortcut: Double bond = 1deg of unsat. 1 triple bond = 2 deg of unsat 1 cyclic molecule = 1 degre of unsat |
||||||||||||||||||
| Functional Groups | BP: Alkoxide>Carboxilic acid> Alcohol>Ether>Alkane BP Amines: Amonium Salt>1ry>3ry Amines basicity is increased with electron donating groups. Secondary amines are stronger bases than primary amines. Imine R=N-H |
|||||||||||||||||||
| Chirality | Geometric Isomers: cis-trans | |||||||||||||||||||
| Stereoisomer: 2^n: same chemical formula. R, S | ||||||||||||||||||||
| Enantiomer R to S, RR to SS, SR to RS | ||||||||||||||||||||
| Diastereomer RS to SS, SR to RR | ||||||||||||||||||||
| Optical Activity: +/- indicate physical bend of light. R/S only indicate imaginary rotation. | Diastereomers and Racemic Mixtures are NOT optically active. | |||||||||||||||||||
| Isomers | Constitutional Isomers: Ortho ./ Para : same molecular formula with different connectivity. Configurational (R), (S): differe in bond configuration. Rotamers: Conformational Isomer. Regioisomer: Different starting material configuration give different structuraly isomeric products. |
|||||||||||||||||||
Induction |
||||||||||||||||||||
| Acidity | S-S-C-P-A-alpha-SP-SP1-SP2-SP3: | "Recall Misscissipah" | ||||||||||||||||||
| Resonance | Contributing structures with delocalized electrons in free p orbitals. The contributing stuctures are not equal. The structure that locates the charge in the more electronegative atom is more stable. Resonance contributes to increasing acidity by stabilizing the negative charge of conjugate bases. |
http://goldbook.iupac.org/R05326.html | ||||||||||||||||||
| REACTIONS | REACTIONS | REACTIONS | ||||||||||||||||||
| Hydrogen | Hydrogen Proton H+, Hydride ion H: -1 | |||||||||||||||||||
| Hydrolysis | ||||||||||||||||||||
| Heterolytic Hydrogen Cleavage | The electron pair of the bond goes to same atom. | |||||||||||||||||||
| Homolytic Hydrogen Cleavage | each electron of the bond goes to a different atom. Radicals are stabilized by electron donating groups. Peroxides give antiMarkovnikov products with alkenes. | |||||||||||||||||||
| Radical Reactions | Initiation Propagation Termination Inhibition Radical Stability: Alllylic and Benzyllic Radical are comparable. Vinyllic Radical has higher bond energy because the double bond in more electronegative. Stereochemical Considerations Br2 vs Cl2: Selectivity; ratio of products |
Good Reference: http://www.mhhe.com/physsci/chemistry/carey/student/olc/graphics/carey04oc/ref/ch10allylic.html http://www.slideshare.net/hussain_761/free-radicals-12023253 |
||||||||||||||||||
| Nucleophilicity | Increases going down the periodic table F- < Cl- < Br-< I-; Increases to the left across a period CH3-> NH2- > OH- Nu: are Lewis Bases. Donate protons.
|
|||||||||||||||||||
| S1 | Solvent: Polar Aprotic DMSO, DMF, MeCN, Dimethyl Ketone, Acetone: Polar Aprotic Solvents stabilize Carbocation. Polar Aprotic solvents cannot bond with Nu: [Adjust], most stable carbocations. Racemization Likely so result is either a racemic mixture or diastereomer. 3ry>2ry: Carbocation is formed. Reaction Rate = k [electrophile] Weak Nucleophiles Favor Sn1
|
Alcohols and Alkyl Halides undergo substitution reactions. | ||||||||||||||||||
| Sn2 | Solvent: Polar Aprotic DMSO,DMF, MeCN, Acetone, Polar Protic Solvents favor E2 over SN2. Polar APROTIC Solvents Favor SN2 over E2. Sn2: Reaction is favored by 1 > 2 > 3 substitution because of crowding in the transition state. All other reactions are 3 > 2 > 1. Transition state is crowded so less substituted molecules undergo reaction faster. The reaction rate = k [nucleophile][electrophile]. Hydrogen bonding solvents prevent the nucleophile from initiating the reaction. STRONG Pointer: Rule Out Possibilities: if you see a protic solvent rule out Sn2... |
References: http://www.masterorganicchemistry.com/2012/12/04/deciding-sn1sn2e1e2-the-solvent/ |
||||||||||||||||||
| E1 | Solvent: Hydrogen Bonding Solvents Reaction Rate = k [Haloalkane]
|
|||||||||||||||||||
| E2 | Polar Protic Solvents favor E2 over SN2. STRONG Base Favors E2 because: base can deprotonate to free a pair of electrons. Reaction Rate = k [Haloalkane] [Base] Antiperiplanar proton
|
Reminder: "Protic = Pi = double bonds use a Pi bond" | ||||||||||||||||||
| Sn1-Sn2-E1-E2 |
Reaction Rate Substrate Stability of Intermediate Strength of Nucleophile Stereochemistry Solvent Rearrangements |
http://www.sparknotes.com/chemistry/organic4/sn1e1/section1.rhtml | ||||||||||||||||||
| Markovnikov | more stable carbocation is formed. H and CH3- migration | |||||||||||||||||||
| antiMarkovnikov | less substituted alkyl bromide bond is formed. | HBr/hv with Peroxides | ||||||||||||||||||
| Hoffman Rule | ||||||||||||||||||||
| Zaitzeff Rule | priority | |||||||||||||||||||
| Molecular Geometry | ||||||||||||||||||||
| Trigonal Bipyramidal | upon loosing a pair of electrons becomes a seesaw. | |||||||||||||||||||
Organic Acids Acidity |
Henderson Hasselbach pH = pKa + log [A-]/[HA] low pKa = High Ka = Strong Acid. If pH < pKa: Protonated. If pH > pKa: BASIC, Unprotonated |
A proton becomes acid when the bond breaks heterolytically. Homolytic Bond Breaking: Homolysis: the bond breaking kcal/mol is higher = requires more energy. Electron pairing in heterolysis stabilizes. |
||||||||||||||||||
| Alkene Reactions | ||||||||||||||||||||
| Alkene HaloAlkane Addition | Markovnikov, Not Enantioselective. | |||||||||||||||||||
| Alkene HaloAlkane Addition with Peroxide | Gives Halogenated less substituted haloalkane. Antimarkovnikov. |
|||||||||||||||||||
| Alkene Hydration Acid Catalyzed | Gives Alcohol Pi bond is protonated initially, forming a carbocation. |
|||||||||||||||||||
| Alkene Oxymercuration/Demercuration | Gives Alcohol. Markovnikov. Pi bond is protonated initially, forming a carbocation. |
|||||||||||||||||||
| Alkene Hydroboration | Give Alcohol Antimarkovnikov. |
|||||||||||||||||||
| Alkene DiHaloxen X-X Attack | Sn2: Trans Product Reaction proceed by instant (-) polarization of pibond by X-X Reaction can yield Halogenated and -OH groups. -OH is Markovnikov. |
|||||||||||||||||||
| Alkene Epoxide Formation | Gives Epoxide and Carboxylic acid.. Followed by acid or base hydrolysis to give Diol. |
|||||||||||||||||||
| Alkene Oxidation with KMnO4 | Gives cis-Diol trans diol with peroxy acid. |
|||||||||||||||||||
| Alkene Hydration (Reduction) (cis or trans) | Gives Alkane. H2 with Ni, Pd, Pt. cis addition. Lindlar Catalyst with dopant stops reaction at alkene. trans addition is with Na, NH3 |
Recall Reduction. | ||||||||||||||||||
| Alkene Ozonolysis | Gives Ketones. Reactant O3 + Zn in H20 |
|||||||||||||||||||
| Aromatic Compounds | Aromaticity: Huckel Number: 4n + 2; Cyclic, free p orbital with a Huckel number, flat and planar. Can be accomplished via Cyclic Delocalization , Resonance, Conjugation If an electron like Oxygen is in the bond we only use one pair of electrons for aromaticity Examples: Purine, Pyridiine, Thymine |
Good Reference: https://www2.chemistry.msu.edu/faculty/reusch/virttxtjml/benzrx1.htm | ||||||||||||||||||
| Aromatic Electrophilic Substitution | The Benzene ring is succeptible to electrophilic attack. Heat is required because the ring is pi stabilized. Ortho/Para: have lone pair of electrons on the atom bonding the ring. with the exception of alkyl R. -NO2 is a META deactivator
Activators have free electrons in the atom bonded to the benzene ring: -NH2, -OC6H5, -OR, -O(-) OrthoPara Deactivators: Cl, Br, I are deactivators |
Deactivators increase anion stability upon protonation: acidity is increased. | ||||||||||||||||||
| Aromatic Diels Alder | Concerted Formation of Ciclic Compounds from conjugated diene. Dienophyle = alkene or alkyne Diastereoselective |
Reference: http://www.organic-chemistry.org/namedreactions/diels-alder-reaction.shtm |
||||||||||||||||||
| Ketone Reactions | Keto/Enol Tautomerism Acid Alpha proton Ketones do not undergo substitution reactions. Beta keto Acid. Beta keto ester. |
Ketone Formation from 2ry Alcohols with Chromate Oxidation. | ||||||||||||||||||
| Ketones: Acetal | Cyclic Contains 2 'ketones' Biologicaly Important. |
|||||||||||||||||||
| Ketones: Hemiacetal | Cyclic Contains 1 ketone Biologicaly Important |
|||||||||||||||||||
| Ketones: Imines | Convert the Carbonyl to an Imine | |||||||||||||||||||
| Ketones: Aldol Condensation | "one ketone stays the same and bonds with a double bond through the alpha carbon, after dehydration, so the carbonyl oxygen of the ketone that does not have alpha hydrogen is lost. study mechanism
|
|||||||||||||||||||
| Acyl Group | Carboxylic Acids> Acyl Halide, Acid Anhydride, Ester, Amide | Acyl derivatives interconvert to lower reactivity. Acid Halide > Acid Anhydride > Ester > Amide less hindered carbonyl is more reactive. |
||||||||||||||||||
| Carboxylic Acids | Acetate CH3 COOR Acidity Order: HC l> Carboxylic Acid > Substituted Phenol > diketone > ketone |
Bases Deprotonate to give COO- Salts | ||||||||||||||||||
Carboxylic Acids: Nucleophilic Addition Elimination |
electronegative groups allow attack at the Carbonyl and serve as good leaving groups. | |||||||||||||||||||
| Carboxylic Acids | Transesterification: Alcohol + Carboxylic Acid gives Ester. | |||||||||||||||||||
| Ester | are neutral in solution. | |||||||||||||||||||
| Ester Hydrolysis | Nucleophilic Acyl substitution : Addition Elimination : Saponification Acid and Basis Catalyzed |
|||||||||||||||||||
| Inorganic Esters | Central Carbon P, S. Contain more than one double bond. | See reaction of PBr3 with 3ROH -> H3PO3 + 3RBr | ||||||||||||||||||
| Amines | Reductive Amination: Amines Attack of Ketones to yield 1,2,3ry substituted Imines that are Reduced with H2/Pd to Amines. Amines to Alkenes via HoffMann Elimination: Less substituted alkenes. |
|||||||||||||||||||
| Amides | Acid Base Reactions with Carboxylic Acids Basic Leaving groups decrease reactitvity of Acid Chlorides with respect to Anhydrides> Esters>Amides Hoffman Rearrangement: Amides are converted to Amines |
|||||||||||||||||||
| Imines | Schiff Base R1R2C=NR3 | |||||||||||||||||||
| BIOCHEMICAL | Sugars, Proteins, Fats, Metabolism, pKa, Anomeric Carbon See SSSFFF Biology |
Good References: http://www.chem.ucla.edu/harding/IGOC/IGOC.html |
||||||||||||||||||
| SUGARS | Fructose, Glucose, Ribose LOOK at chirality FARTHEST from C1 to determine L/R. Sucrose: Glc + Fru: Alpha-1,2 Lactose: Gal + Glc: Beta-1,4 Maltose: Glc + Glc: Alpha-1,4 Cellobiose: Glc + Glc: Beta-1,4 Glycogen: α 1-4 |
EPIMERS are NEVER Enantiomers. Epimers differ only in one stereogenic center that IS NOT the last chiral carbon. Alpha/Beta epimers: Alpha = Axial, Beta = Equatorial HAWORTH Projection: Beta is UP!!! |
||||||||||||||||||
Monosaccharides Sugars |
D, L Aldohexose vs ketopentose, vs aldotriose (threeOHgroups) ketohexoses (6OH and 1 C=O) Anomeric Carbon: cyclic: opposite the CH2OH outside the rinh C1 orientation |
|||||||||||||||||||
| Hexoses | Glucose | |||||||||||||||||||
| Pentoses | Xylose Ribose |
|||||||||||||||||||
| Sulfur Containing Molecules | Mesylate, Triflate, Tosylante | |||||||||||||||||||
| AMINOACIDS | All natural AA are L, amphoteric Carboxylic pKa aprox 2. Amino Group pKa aprox 10 |
|||||||||||||||||||
| Extraction | Form salt to become water soluble: Carboxilic acids are extracted with weak bases. NaHCO3 is better than NaOH because it destabilized aromatic structures.
|
|||||||||||||||||||
| 10 Enzymes Everyone should know | Glycogen Phosphorylase Phosphoglucomutase Glucose 6-Phosphatase ATPase Hexokinase/ Hexose / Dehydrogenase / Oxidase Amylase Galactosydase CYP450 Cytochrome Oxidase
|
|||||||||||||||||||
| Reactant List | HNO3 | EAS Activator of | ||||||||||||||||||
| Br2 + FeBr3 (heat) | EAS Bromination | |||||||||||||||||||
| H2SO4 (heat) | EAS Sulfonation | |||||||||||||||||||
| Friedel-Craft RCOCl + AlCl3 (heat) | EAS Aryl Ketone | |||||||||||||||||||
| Friedel-Craft RCl + AlCl3 (heat) | EAS Alkylation | |||||||||||||||||||
| Hg(OAc) in Water then NaBH4 | Alkene Oxidation to Markovnikov Alcohol | |||||||||||||||||||
| BH3 in THF then H2O2 | Alkene Anti Markovnikov Alcohol | |||||||||||||||||||
| KMnO4 | cis Diol | |||||||||||||||||||
| OsO4 | cis Diol | |||||||||||||||||||
| PCC | Anhydrous Oxidant: 1ry Alcohol to Aldehydes | |||||||||||||||||||
| CrO3/ H2CrO4/Cr2O7-2/CrO3 | Aqueous Oxidants: 2ry Alcohol to Ketones | |||||||||||||||||||
NaBH4 in EtOH |
Reductor of Carbonyl | |||||||||||||||||||
| LiAlH4 in Ether (aprotic) | Reductor of Carbonyl | |||||||||||||||||||
| NaH, KH | Hydride bases | |||||||||||||||||||
| Grignard RMgBr in Acid | Reductor of Carbonyl + R | |||||||||||||||||||
| RLi | Reductor of Carbonyl + R | |||||||||||||||||||
| Wittig Phosphonium Ylide | Reductor of Carbonyl to Alkene | |||||||||||||||||||
| NaOH/H2O Acid | Aldol Condensation. Base attacks Alpha proton. Beta Hydroxyl Carbonyl or Alpha/Beta unsaturated Carbonyl |
|||||||||||||||||||
| NaOH / KOH | Saponification | |||||||||||||||||||
| SOCl2 | Inorganic Ester reactions. | |||||||||||||||||||
| PBr3 | ||||||||||||||||||||
| NaHCO3 | Extraction of Carboxilic Acids | |||||||||||||||||||
| 10% HCl | Extraction of Amines | |||||||||||||||||||
| Tosyl Chloride | Stabilizez anion charge in COO-, H leaves | |||||||||||||||||||
| Michael | ||||||||||||||||||||
| Wolf-Kishner Reduction | ||||||||||||||||||||
| Molecules to know | Furan, Pyran, Toluene, Naphtalene, Phenanthrene | |||||||||||||||||||
| Polymer Chemistry | ||||||||||||||||||||
| Elastic Properties | Elastic Limit | |||||||||||||||||||
| Thermal Expansion Coefficient | ||||||||||||||||||||
| Shear | ||||||||||||||||||||
| Compression | ||||||||||||||||||||
SEPARATION TECHNIQUES |
||||||||||||||||||||
| DISTILLATION | ||||||||||||||||||||
| Short Path Distillation | ||||||||||||||||||||
DETECTION TECHNIQUES |
||||||||||||||||||||
| Detection Techniques | Detection IR | |||||||||||||||||||
IR cm^-1 |
3600-3200 -OH 3300 Amines (OH broadest, NH sharper) 3000 C-H 2300 - 2100 C---C or C---N 1700 C=0 (Carbonyl, Carboxylic Acids) 1650 C=C |
|||||||||||||||||||
CHROMATOGRAPHY |
||||||||||||||||||||
| Chromatography | Separation of derivatized diastereomers. Enantiomers can be reacted with a known stereochemistry comp. | |||||||||||||||||||
GAS Chromatography |
FID TCD ECD NPD |
|||||||||||||||||||
Wet Column Chromatography |
Also referred to as Gravity Column Chromatography https://www.youtube.com/watch?v=HGGT5Mo-wPI |
|||||||||||||||||||
Planar Chromatography |
TLC R factor: Polar molecules are retained by Silica, so have smaller R f. | |||||||||||||||||||
| Size Exclusion Chromatography SEC | Large Elute First. |
Size Exclusion, gel permeation chromatography or gel filtration chromatography. separates particles on the basis of molecular size (actually by a particle's Stokes radius). It is generally a low resolution chromatography and thus it is often reserved for the final, "polishing" step of the purification. It is also useful for determining the tertiary structure and quaternary structure of purified proteins. SEC is used primarily for the analysis of large molecules such as proteins or polymers. SEC works by trapping these smaller molecules in the pores of a particle. The larger molecules simply pass by the pores as they are too large to enter the pores. |
||||||||||||||||||
|
SDS-PAge |
|
|||||||||||||||||||
|
Scintillation counting |
Iodomelatonin in the presence of selective antagonist for the receptor We have pharmacologically characterized recombinant human mt1 and MT2 receptors, stably expressed in Chinese hamster ovary cells (CHO-mt1 and CHO-MT2), by measurement of [3H]-melatonin binding and forskolin-stimulated cyclic AMP (cAMP) production. [3H]-melatonin bound to mt1 and MT2 receptors with pKD values of 9.89 and 9.56 and Bmax values of 1.20 and 0.82pmolmg−1 protein, respectively. Whilst most Whilst most melatonin receptor agonists had similar affinities for mt1 and MT2 receptors, a number of putative antagonists had substantially higher affinities for MT2 receptors, inclu ding luzindole (11 fold), GR128107 (23 fold) and 4-P-PDOT (61 fold). In both CHO-mt1 and CHO-MT2 cells, melatonin inhibited forskolin-stimulated accumulation of cyclic AMP in a concentration-dependent manner (pIC50 9.53 and 9.74, respectively) causing 83 and 64% inhibition of cyclic AMP production at 100nM, respectively. The potencies of a range of melatonin receptor agonists were determined. At MT2 receptors, melatonin, 2-iodomelatonin and 6-chloromelatonin were essentially equipotent, whilst at the mt1 receptor these agonists gave the rank order of potency of 2-iodomelatonin>melatonin>6-chloromelatonin. In both CHO-mt1 and CHO-MT2 cells, melatonin-induced inhibition of forskolin-stimulated cyclic AMP production was antagonized in a concentration-dependent manner by the melatonin receptor antagonist luzindole, with pA2 values of 5.75 and 7.64, respectively. Melatonin-mediated responses were abolished by pre-treatment of cells with pertussis toxin, consistent with activation of Gi/Go G-proteins. |
|||||||||||||||||||
SPECTROSCOPY |
||||||||||||||||||||
| Mass Spectroscopy | Base Peak is the largest peak. It is fragmented. Parent peak is the ion of the molecule without fragmentation. Uncharged molecules are not detected. More fragmentation produces smaller peaks. Quantitative and Qualitative: The m/z ratio |
|||||||||||||||||||
| Nuclear Magnetic Resonance | Shielding/Deshielding Electron donating are shielding, Electron Withdrawing are deshielding. Left axis has larger number. So deshielded protons appear to the left=downfield (-RCOOH). Shielded protons appear to the right (H-C-CH3x3) 1-propyne vs 1 propene: s haracter is less shielding. ORDER: Aldehyde and Carboxylic- Aromatic- Vinillyc--- Halogen, ether, allyl, alkyne, benzyllic . |
|||||||||||||||||||
| H NMR | Allylic proton is very deshielded. Memorize the 6-8ppm region is Aromatic. Geminal Methyl Groups are identical. |
Good Reference: http://www.stolaf.edu/depts/chemistry/courses/toolkits/380/js/nmr1/ |
||||||||||||||||||
| C NMR | Height of Peak proportional to number of H attached to C. | |||||||||||||||||||
BIOCHEMICAL TECHNIQUES |
||||||||||||||||||||
| BIOCHEMICAL TECHNIQUES | BIOCHEMICAL TECHNIQUES | BIOCHEMICAL TECHNIQUES | ||||||||||||||||||
| ELISA | Enzyme Linked Immuno Sorbent Assay Antibody binds antigen-Wash-Enzume Linked antibody-Wash-Color Change |
|||||||||||||||||||
| VULCANIC CHEMISTRY | BIOCHEMICAL TECHNIQUES | |||||||||||||||||||
Vulcanic Chemistry
|
Tectonic Plates Nazca Plates Subduction Hotspots, Magma, 700C to 1600C, SiO2, Fe, Mg, 2200-2800kg/m3, IDDP magma geothermal energy generator Phreatic vs Phreatomagmatic vs Magmatic Eruption Magmatic Eruption: Decompression of Gas Phreatic Eruption: Superheated Water Expansion Phreatomagmatic Eruption: Compression of Gas within Magma Hawaiian<Strombolian<Pelean<Plinian<UltraPlinian Mount Pele in St Pierre, Martinique exploded in 1903 killing 30k. |
Tools for Earthquake Prediction: Thermal tools: Heat Chemical: Vulcanic Chemistry, Water Quality and Gas Composition Nuclear: Particle Counters through volcanic Mass Mechanical: Earthquakes and Sound waves. |
||||||||||||||||||